Background
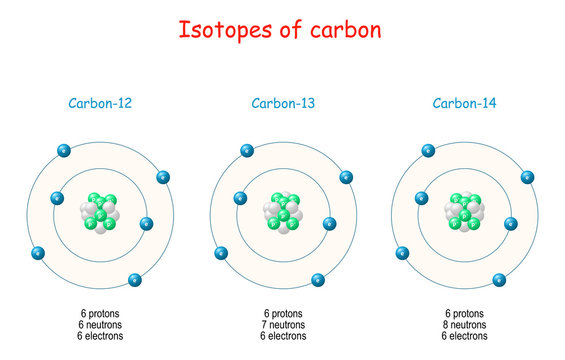
All isotopes of radium are highly radioactive, with the most stable isotope being radium-226, which has a half-life of 1600 years and decays into radon gas (specifically the isotope radon-222). When radium decays, ionizing radiation is a product, which can excite fluorescent chemicals and cause radioluminescence. Carbon is found in all living things and is the building block for organic material, there is carbon 12, 13 and 14 and they all have different properties. This isotope was discovered on February 27, 1940 by Martin Karmen and Sam Ruben and is the most radioactive out of all carbon isotopes.
14C is a radioactive isotope of carbon. It was discovered in 1934 by Grosse as an unknown activity in the mineral endialyte. In the same year, Kurie (Yale) exposed nitrogen to fast neutrons and observed long tracks in a bubble chamber. He had produced 14C. It was in atmospheric CO2 by Libby in 1946. He determined the half life to be 5568 years. This half life has later been re-determined by Godwin. The new half life is 5730 years. Libby recognized that due to its occurrence in natural materials, 14C can be used as a dating tool for materials that contain carbon compounds derived from atmospheric CO2 either by simple mixing processes or by carbon exchange. The mean life time of roughly 8000 years is ideal for dating of reservoirs that are a few decades to a few ten thousand yeas old. For groundwater, this means that 14C dating can be applied to aquifers that contain water formed during periods that reach well into the past glacial time. 14C is a widely used tool to establish chronologies for groundwater flow systems and climate records for the Holocene and Pleistocene. It is considered to be the most important tool for age dating of ‘old’ groundwater.
14C can be used as a dating tool for materials that contain carbon compounds derived from atmospheric CO2 either by simple mixing processes or by carbon exchange. The mean life time of roughly 8000 years is ideal for dating of reservoirs that are a few decades to a few ten thousand yeas old. For groundwater, this means that 14C dating can be applied to aquifers that contain water formed during periods that reach well into the past glacial time. 14C is a widely used tool to establish chronologies for groundwater flow systems and climate records for the Holocene and Pleistocene. It is considered to be the most important tool for age dating of ‘old’ groundwater. The challenge in 14C dating of groundwater is the determination of the initial 14C content of groundwater at the time of recharge, i.e., at the time when groundwater is isolated from exchange with the soil air and moves away from the water table. Electrax crack mac.
Lexmark x3550 driver windows 10. There is also a stable isotope of carbon, 13C. This isotope is important in that it allows us to correct for carbon isotope fractionation in nature and during analytical procedures.
Isotopes Of Carbon
Abundance of carbon isotopes in nature
| 12C | 13C | 14C |
| 98.89 % | 1.11 % | ~10-12 |
13C measurements are reported in the d13
 C notation relative to a standard (PDB, or the newer VPDB standard, considered identical to PDB)
C notation relative to a standard (PDB, or the newer VPDB standard, considered identical to PDB) Isotope ratios are typically measured by mass spectrometry
d13C values cover a wide range in nature (Fig) influenced by fractionation processes analogue to what we discussed in the water isotope section
14C activities are referred to an international standard, known as as 'modern carbon'
Natural 14C production
14C is mainly produced by interaction of cosmic ray derived secondary neutrons with 14N in the atmosphere.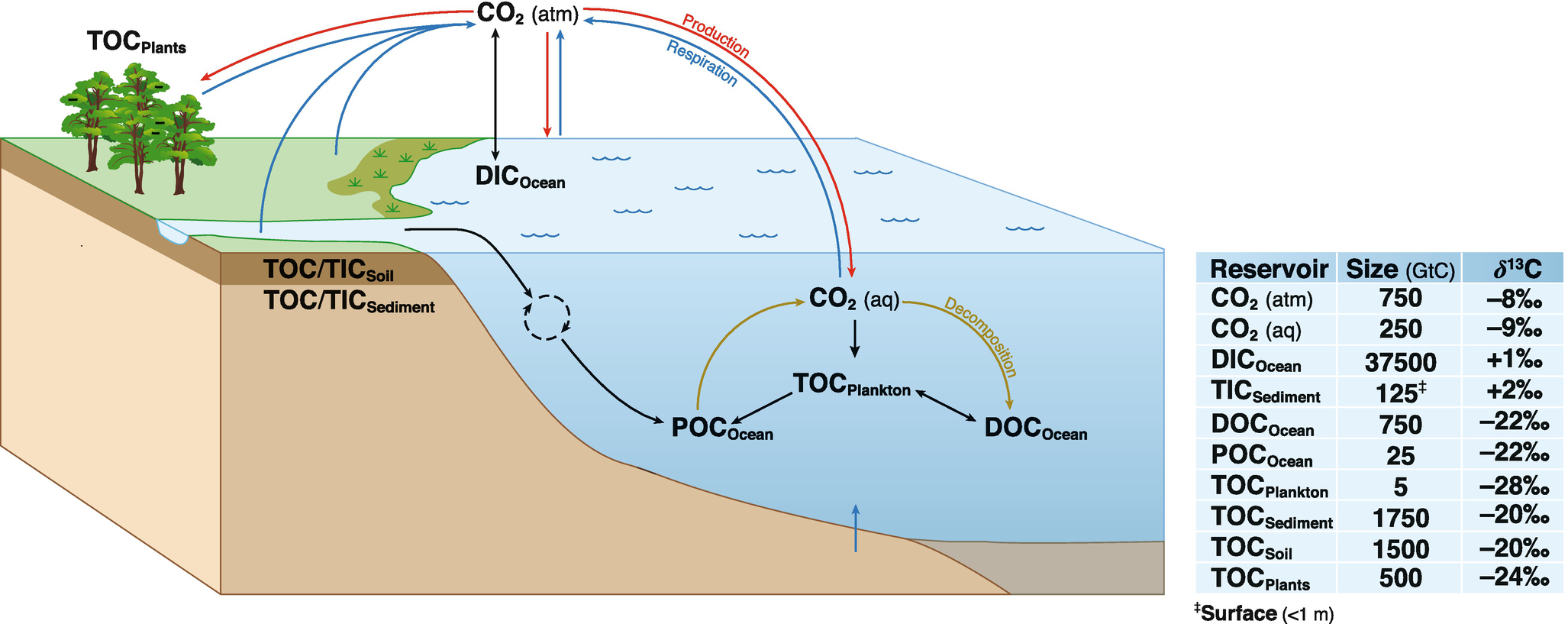 the production rate is 2.4 ± 0.2 atoms (cm2 sec)-1
the production rate is 2.4 ± 0.2 atoms (cm2 sec)-1The production rate has not been constant over time, comparison with tree ring chronologies and corals have shown variations in the production rate which result in an offset between calendar and 14C ages of thousands of years (Fig) (Fig)
Radioactive decay
14C decays by b- decay with a maximum energy of 0.158 MeV.146C -> 147N+ e- + anti-neutrino+ Q
Its half life t is 5730 years, i.e., somewhat larger than the half life determined by Libby (5568 ys). Differnt fields tend to use different half lifes.
Natural global inventory
The global inventory of natural 14C is about 75 tons. The specific activity in pre-industrial times was 13.56 dpm (gC)-1. dpm stands for decay per minute.
Anthropogenic 14C production
The main source of anthropogenic 14C is so-called ‘bomb’ 14C, i.e., 14C produced during atmospheric testing of nuclear weapons. At the peak of surface testing of nuclear devices in 1963, the atmospheric 14C activity had reached about twice that of natural 14C (Fig). The bomb 14C has been produced by interaction of atmospheric nitrogen with the high neutron flux from the explosion of nuclear devices (mainly thermonuclear devices). Local increases in atmospheric 14C have been observed in the vicinity of nuclear power plants.
Before bomb production began, 14C (and 13C) dropped due to anthopogenic emisssions of fossil carbon (Suess effect, Fig)
Notation
The notation of 14C activities is discussed in detail in Stuiver and Pollach (Radiocarbon, 19, 355-365, 1977). In short, 14C is calibrated against an NBS (National Bureau of Standards) oxalic acid standard. The internationally accepted radiocarbon dating reference is 95% of the activity, in 1950 AD, of the NBS oxalic acid normalized to d13C of –19.3‰ with respect to PDB. The factor of 0.95 adjusts the oxalic acid to the activity of wood from 1840 to 1860 (‘pre-industrial’). Results are reported in pmC (% modern carbon).A0N = 0.95 AOX [1 – 2.3(d13C + 19.3)/1000]
- AON: 14C activity of oxalic acid normalized for 14C fractionation
- AOX: 14C activity of oxalic acid
- The d13C correction of 19‰ takes into account the fractionation of 14C during the combustion of oxalic acid.
Isotopes Of Carbon 12
Generally radiocarbon concentrations of samples are normalized to a common d13C value of -25‰. The fractionation for 14C is 2.3 times the fractionation for 13C and therefore the enrichment in 14C due to a factionation amounts to:2.3(d13C + 25‰)
For groundwater studies, the pmc (percent modern carbon) notation is used.
pmc = (ASN/Aabs) 100% = ASN [AON el(y-1950)]-1 100%
- ASN: activity of sample normalized for fractionation using d13C
- AON: 13C normalized activity of oxalic acid
- Aabs: Absolute 14C activity of the sample
- y: year of measurement of oxalic acid
14C dating
Principle:In the atmosphere, 14C is incorporated into 14CO2 and takes part in the global carbon cycle. It is assimilated by plants. Except for isotope fractionation, 14C in living organic matter is the same as that in atmospheric CO2. After organic matter dies, the 14C concentration decreases due to radioactive decay. If there is no isotope exchange, radioactive decay is the only 14C sink and if the initial 14C activity is known, an age can be calculated from the measured 14C activity of a sample.
In groundwater applications typically DIC (dissolved inorganic carbon; DIC = CO2(aqueous) + HCO3- + CO32-) is extracted from the water a and its measured 14C activity is compared to the initial 14C activity. Determination of the initial 14C activity can be challenging and typically requires correction models that account for the carbon chemistry in the unsaturated and saturated soil zones.
14C(t) = 14C(t0) e-l (t-t0)
ln 14C(t) = ln 14C(t0) (-l (t-t0))
ln [14C(t)/14C(t0)] = -l (t-t0)
D t = (t - t0) = -l-1 ln [14C(t)/14C(t0)] = T1/2 / ln2 {ln [+C(t0)/14C(t)]}
l: radioactive decay constant of 14C: l = t-1; t : mean life time: t = T1/2/ln2

See summary in Clark and Fritz (chapter 8) for details
In short, the 14C activity of DIC (dissolved inorganic carbon) in groundwater is determined by the following factors: (Fig. 8.6 in Clark and Fritz):
- activity in soil CO2 (soil air and root respiration); activity » 100 pmc
- dissolution of carbonates (lime stone); activity » 0 pmc
- dissolution of carbonates can occur in the unsaturated soil zone (open system) or in the saturated soil zone (closed system).
- isotope exchange can lead to a decrease in 14C activity in addition to radioactive decay
- a variety of models can be used to estimate the initial 14C activity in groundwater. They include the very simple ‘Vogel’ model, several models that correct for carbon chemistry using chemical or 13C balances, and the complex NETPATH model by Plummer. The latter accounts for the chemical evolution of the groundwater along flowpaths.
completely open system: 14C activity: » 100 pmc
completely closed system: 14C activity: » 50 pmc
Chemical and isotopc evolution in recharge zone: (Fig) (Fig) Open and closed system conditions
‘real world’ systems are somewhere in between open and closed and the correction models mentioned above and described in Clark and Fritz (chapter 8) have to be applied.
Measurement- Technique: low-level b - counting using gas-filled proportional counters or AMS (Accelerator mass spectrometry)
- Water sample size: ca. 100 liters (low-level counting) or several hundred ml (AMS)
- Measurement precision: ± 0.2 to ± 1% (1-s error) for concentrations close to 100 pmc. This corresponds to an age resolution of about ± 16 to ± 40 years.
- Detection limit: <1% modern corresponding to ages of about 40000 years.
Resources
- Fairbanks, R. G., Mortlock, R. A., Chiu, T. C., Cao, L., Kaplan, A., Guilderson, T. P., Fairbanks, T. W., Bloom, A. L., Grootes, P. M., and Nadeau, M. J. (2005). Radiocarbon calibration curve spanning 0 to 50,000 years BP based on paired Th-230/U-234/U-238 and C-14 dates on pristine corals. Quaternary Science Reviews 24, 1781-1796.
- Reimer, P. J., Baillie, M. G. L., Bard, E., Bayliss, A., Beck, J. W., Bertrand, C. J. H., Blackwell, P. G., Buck, C. E., Burr, G. S., Cutler, K. B., Damon, P. E., Edwards, R. L., Fairbanks, R. G., Friedrich, M., Guilderson, T. P., Hogg, A. G., Hughen, K. A., Kromer, B., McCormac, G., Manning, S., Ramsey, C. B., Reimer, R. W., Remmele, S., Southon, J. R., Stuiver, M., Talamo, S., Taylor, F. W., van der Plicht, J., and Weyhenmeyer, C. E. (2004). IntCal04 terrestrial radiocarbon age calibration, 0-26 cal kyr BP. Radiocarbon 46, 1029-1058.
- Levin, I. and Hesshaimer, V. (2000) Radiocarbon - a unique tracer of global carbon cycle dynamics. Radiocarbon, 42, 1, 69-80.
- Fontes, J. C.; Garnier, J. M. (1979) Determination of the initial 14C activity of the total dissolved carbon; a review of the existing models and a new approach. Water Resour. Res., 15, 399-413.
- Mook, W.G. (1980) Carbon-14 in hydrogeological studies. In: Handbook of environmental isotope geochemistry (Fritz, P, and Fontes, J.C., editors), Vol 1, Elsevier Sci. Publ. Co., Amsterdam, 49-74.
- Plummer, L.N., E.C. Prestemon, and D.L. Parkhurst (1991) An interactive code (NETPATH) for modeling net geochemical reactions along a flow path. USGS Water-Resources Investigations Report,} 91-4078, USGS, Reston.
- Plummer, L.N., Prestemon, E.C., and Parkhurst, D.L., 1994, An interactive code (NETPATH) for modeling NET geochemical reactions along a flow PATH--version 2.0: U.S. Geological Survey Water- Resources Investigations Report 94-4169, 130 p.
- NETPATH home page
- Levin I, Schuchard J, Kromer B, Münnich KO (1989) The continental European Suess effect. Radiocarbon 31(3):431–40.
