The Crossword Solver found 20 answers to the Positively charged particle crossword clue. The Crossword Solver finds answers to American-style crosswords, British-style crosswords, general knowledge crosswords and cryptic crossword puzzles. Enter the answer length or the answer pattern to get better results. Click the answer to find similar crossword clues. A positively charged atom is called a cation and a negatively charged atom is called an anion. Thus, depending on the surface charge, we can classify any particle as either positively charged.
- Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
- The nucleus was discovered in 1911, as a result of Ernest Rutherford's efforts to test Thomson's 'plum pudding model' of the atom. The electron had already been discovered earlier by J.J. Thomson himself.
- In general, an atom with a charge, either positive or negative, is called an ion.A positively charged atom is called a cation and a negatively charged atom is an anion.
An ion is an atom or group of atoms where the number of electrons is not equal to the number of protons. Electrons have a negative charge, whereas protons have a positive charge. When an atom gains electrons, this results in a negative charge. This type of ion is called an anion. When an atom loses electrons, this results in a positive charge. A positively charged ion is called a cation. Let's explore several ion examples of both types.
Examples of Positive Ions
Positive ions are typically metals or act like metals. Many common materials contain these ions. Mercury is found in thermometers, for instance, and aluminum is a metal that is found in a surprising amount of things. It's even an ingredient in baking soda and in certain other food products!
DDJ-200 & WeDJ Tutorials Develop your DJ skills. Find out how to set up the controller and software, play tracks, and express yourself by putting your own spin on the music. Become a master of the DDJ-200 and WeDJ with this tutorial series. Set everything up. DDJ-200. DDJ-WeGO4 DDJ-WeGO3.DDJ-200 Transition FX is not supported. Main features Make the music your own. FX (effects): Change the texture of the sound using. 
Positively Charged Atomic Particles Are Called
The positive charge (more protons versus electrons) for a cation is shown by a number and plus sign after the formula. If there's just a plus sign, it means the charge is plus 1. Some examples of cations, or positive ions, include the following:
- Aluminum - Al+3
- Barium - Ba+2
- Bismuth - Bi+3
- Cadmium - Cd+2
- Calcium - Ca+2
- Cesium - Cs+
- Chromium (III) - Cr+3
- Cobalt - Co+2
- Copper (I) - Cu+
- Copper (II) - Cu+2
- Hydrogen - H+
- Iron (II) - Fe+2
- Iron (III) - Fe+3
- Lead (II) - Pb+2
- Lithium - Li+
- Magnesium - Mg+2
- Mercury (I) - Hg2+2
- Mercury (II) - Hg+2
- Nickel - Ni+2
- Potassium - K+
- Rubidium - Rb+
- Silver - Ag+
- Sodium - Na+
- Strontium - Sr+2
- Tin (II) - Sn+2
- Zinc - Zn+2
Examples of Negative Ions
Just as atoms can lose electrons to become cations, some can gain electrons and become negatively charged anions. Again, you may be familiar with some of these ions. Fluoride is sometimes added to community water supplies. A million little pieces. Your dentist may also give you a flouride treatment.
The negative charge (fewer protons than electrons) for an anion is shown by a number and minus sign after the formula. If there's just a minus sign, it means the charge is minus 1. Here are several examples of anions:
- Bromide - Br-
- Chloride - Cl-
- Fluoride - F-
- Iodide - I-
- Nitride - N3-
- Oxide - O2-
- Sulfide - S2-
Polyatomic Cations and Anions
If an ion consists of two or more atoms it is called a polyatomic ion. Just like their single-atom counterparts, they too can gain and lose electrons.
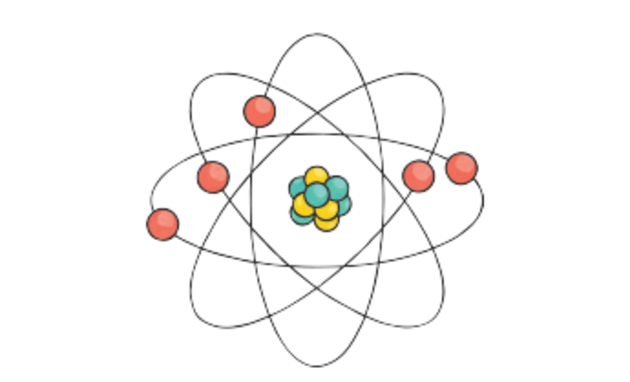
Polyatomic Cations
Ions with multiple atoms that lose electrons, and are thus positively charged, are called polyatomic cations.
Positively Charged Atom

- Ammonium - NH+4
- Hydronium - H3O+
Polyatomic Anions
Ions with multiple atoms that gain electrons, and are thus negatively charged, are called polyatomic anions. In the list below, the charge has been put in parentheses for ease of legibility, but standard notation calls for the charge to be written as a superscript instead.
- Acetate - CH3COO- or C2H3O2-
- Arsenate - AsO43-
- Bicarbonate or hydrogen carbonate - HCO3-
- Borate - BO33-
- Carbonate - CO32-
- Chlorate - ClO3-
- Chlorite - ClO2-
- Chromate - CrO42-
- Cyanide CN-
- Dichromate - Cr2O72-
- Dihydrogen phosphate - H2PO4- or H2O4P-
- Formate - CHO2- or HCOO- or CHOO-
- Hydrogen sulfate or bisulfate - HSO4-
- Hydrogen sulfite or bisulfite - HSO3-
- Hydrogen phosphate - HPO42-
- Hydroxide OH-
- Hypochlorite - ClO-
- Nitrate - NO3-
- Nitrite - NO2-
- Oxalate - C2O42-
- Perchlorate - ClO4-
- Permanganate - MnO4-
- Peroxide O22-
- Phosphate - PO43-
- Phosphite - PO33-
- Silicate - SiO32-
- Sulfate - SO42-
- Sulfite - SO32-
- Thiocyanate - SCN-
- Thiosulfate - S2O32-
Ionic Compounds
An ionic compound is made up of one or more anions and one or more cations.
Some examples of ionic compounds include:
- Aluminum sulfide - Al2S3
- Beryllium chloride - BeCl2
- Boron iodide - BI3
- Calcium nitride - Ca3N2
- Copper phosphide - Cu3P
- Iron (II) iodide - FeI2
- Iron (III) oxide - Fe2O3
- Lead (II) sulfide - PbS
- Lead (IV) phosphide - Pb3P4
- Lithium fluoride - LiF
- Magnesium chloride - MgCl2
- Potassium bromide - KBr
- Sodium fluoride - NaF
- Sodium nitride - Na3N
Fully Charged Reaction
When you study chemistry, you will encounter many examples of ions, as well as the different types of ions and how they interact and relate to each other. For more on the topic, be sure to explore some examples of chemical bonds and examples of chemical properties. Perhaps they'll be the catalyst for positive change in your learning experience!
